Grace Cary/Getty Images
The Atomic Spectroscopy Group at the National Institute of Standards and Technology, which provides definitive measurements integral to developing biomedical instrumentation that helps diagnose health conditions and advancing microchips, will close in a few weeks as part of NIST-wide eliminations.
However, a new campaign aimed to express to the Trump administration how detrimental this would be to advancing medical care has garnered the attention and support of scientists worldwide.
WHY IT MATTERS
NIST's Atomic Spectroscopy Group "provides accurate reference data on spectral lines and energy levels for a wide variety of important applications," according to its government website.
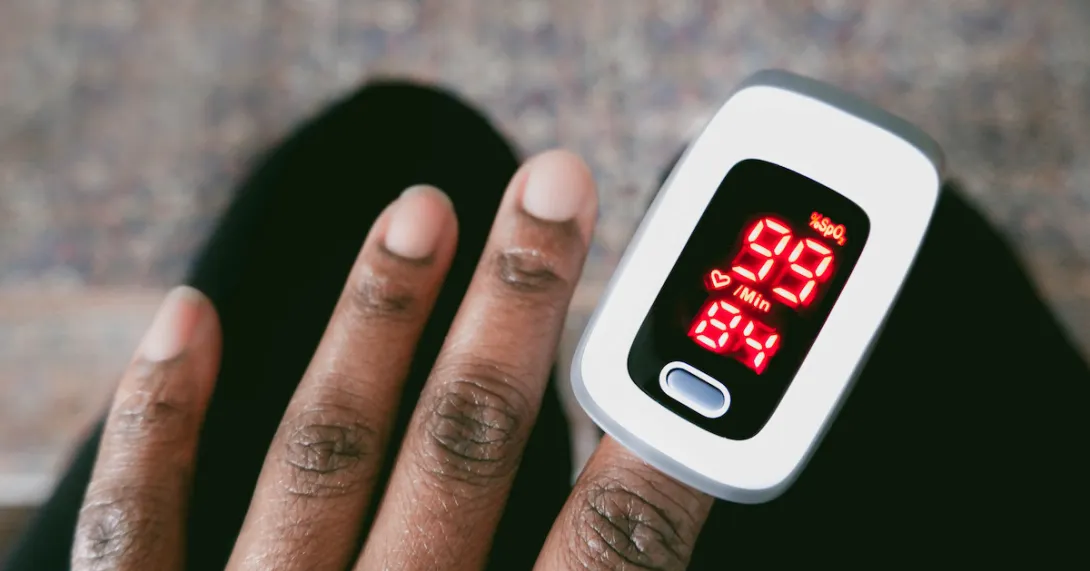
These "atomic fingerprints" define the state of all kinds of plasma, including the oxygen level in a patient's blood as measured through devices like pulse oximeters.
Yuri Ralchenko, the lab's director, sent a letter to scientists notifying them that all staff was expected to be laid off by mid-April, according to a report Wednesday in National Public Radio.
The action has been attributed to cuts by the new Department of Government Efficiency, or DOGE, a department now so active across the federal government that some have turned its actions into a newly-coined verb: "I doge / You doge / She doges / They doge."
Since the lab has been "doged," a petition posted to Change.org by Evgeny Stambulchik asked for signatures to oppose the layoff of the NIST Atomic Spectroscopy Group and has garnered 2,500 signatures as of reporting time. There is also a campaign website.
"The precise measurements of spectra from neutral atoms to highly charged ions have helped to discover many new exoplanets, accurately measure nuclear radii and develop new powerful diagnostic techniques," the petition said.
Stambulchik, who is a physicist at the Weizmann Institute of Science in Israel, reportedly told NPR that if the spectroscopy group closes, researchers worldwide will waste hours hunting for the best spectral measurements.
Those measurements ultimately expand patient access to diagnostics, imaging technologies and treatments.
Nobel Prize-winning physicist Sheldon Glashow and numerous scientists have signed the petition, according to the story.
Of note, the development of medical devices is also facing increased cost with President Donald Trump's tariffs.
AdvaMed, a medical device trade association, requested a carve-out exemption for the medical device industry for tariffs on medical products from Canada, Mexico and China in February.
"We have shared with the Administration our concerns about the potential impact tariffs could have on the medical technology supply chain that American patients depend on for their care," Scott Whitaker, AdvaMed's president and CEO, said in a statement.
THE LARGER TREND
In early January, the U.S. Food and Drug Administration published updated draft recommendations to help improve the performance of pulse oximetry devices, which use spectrophotometry to determine the proportion of hemoglobin saturated with oxygen across skin tones.
A 2023 lawsuit to halt the sale of flawed pulse oximeters by the Roots Community Health Center in Oakland, California, cited concerns about devices that gave erroneous readings about the oxygen levels in patients with darker skin.
The FDA then warned the healthcare sector about potential risks of inaccuracy and said it became aware that pulse oximeters could be less accurate in people with dark skin pigmentation.
The FDA's regulatory update proposed in 2024 – Pulse Oximeters for Medical Purposes – Non-Clinical and Clinical Performance Testing, Labeling and Premarket Submission Recommendations – was to sharpen recommendations for gathering real-world and laboratory clinical data to evaluate device performance accuracy across the range of skin pigmentations.
Initially removed from parts of the FDA's website under the diversity, equity and inclusion scrub, the regulatory docket currently lists the comment period closed as of March 10 with 70 comments.
ON THE RECORD
"I cannot believe that the government would be stupid enough," Glashow reportedly said in a video statement sent to NPR.
Andrea Fox is senior editor of Healthcare IT News.
Email: afox@himss.org
Healthcare IT News is a HIMSS Media publication.


